Update - 01/27/2021
The full update is long, so here is a bullet point summary:
~ We have successfully produced functional insulin and C-peptide using Physarum polycephalum. We have also designed a device that, with more development, could allow people to produce their own safe, functional insulin at home.
~ We have discovered that dysfunction in the traditional medication commercialization process and market structure is to blame for high medication prices, especially insulin.
~ Introducing our PhyR technology to the market via the traditional route would have little to no effect on insulin prices because manufacturing costs are not the problem. Instead, we have created a new and somewhat radical solution that is achievable by individuals or a small group with few resources and could produce effective, very low cost insulin.
~ We are hesitant to proceed with the new solution because it's unclear if people want it. For that reason, we have published our work, technology, and plans here (in the information packet link below) so anyone who wants to use it can, and so we can get feedback from you.
~ Please message or email me feedback on our research and technology; we would like to hear your opinions on if the solution we've proposed could be helpful and viable.
Dear supporters,
Thank you so much for your continuing generosity and encouragement. You’ve helped sustain this project for almost two years even in the midst of struggles brought on by a global pandemic. I cannot express how grateful I am for you and how I respect your strength.
During these last two years, I and several volunteers and team members have put your donations to work in developing the theoretical technology I described in April of 2019. We have had the opportunity to present the technology many times across the country and even internationally, including events at Brigham Young University, the University of Utah, the National University of Singapore, and with many groups of individual supporters. It seems like we’re met with almost overwhelming encouragement and support everywhere we mention the goals of this project. Nearly everyone I’ve personally encountered believes that medication and care are too expensive and that change is necessary in the American healthcare system, especially for those with diabetes.
The goal of The PhyR Project has always been to develop innovative technology with the intention of driving down the cost of insulin and providing the other necessary proteins that current insulin lacks. I am ecstatic to tell you that we have successfully produced insulin and those other proteins using Physarum polycephalum and we have designed a system (the PhyR machine) capable of capturing, purifying, and preparing that insulin for use in the human body to treat type 1 and type 2 diabetes. Even more excitingly, the system we have designed could potentially be used by an individual to produce their own insulin at home, affordably, and independent of insurance and pharmaceutical companies. This system requires more extensive development before it can be trusted to produce insulin safely and reliably at home, but we have proven that it is possible.
While developing this technology, we also dedicated significant time and resources to determining what has gone wrong in the US healthcare system to allow insulin and other medications to cost so much. We found what we believe is the answer and published our analysis in our presentation packet for the University of Utah’s Bench to Bedside program. The packet is included below for public access and I encourage anyone interested to read it carefully; it contains the sum of our work and research over the last two years. In short, we discovered that manufacturing costs have little to no relation to insulin list prices. From section 2.2.3 of the packet:
“...assume a technological advancement reduced the cost of producing, purifying, validating, and packaging insulin by half ($15 per vial). Implementation of that advancement would reduce the list price of insulin from $275 per vial to $260 per vial. If a similar advancement reduced the manufacturing cost to a tenth of the original ($3 per vial), it would lower the list price to $248. If it was reduced to one hundredth of the original cost ($0.3 per vial), the price would be lowered to $245.75. Finally, if it was somehow possible to produce, purify, validate, and package insulin with no manufacturing costs whatsoever, the consumer would still pay $245.45 for a single vial of insulin in the current system. As the system currently exists, no degree of innovation will have any power to significantly reduce the cost of insulin or most other therapeutic drugs because manufacturing cost contributes so little to the actual price the consumer pays.”
As you know, the PhyR technology we’ve been developing is a manufacturing innovation that could significantly reduce the cost of making insulin. Unfortunately, as the above excerpt details, we cannot significantly affect the list price of insulin through manufacturing innovation because the price is being driven up by other factors.
Factors and companies that directly inflate the list price of insulin are discussed in detail in section 2.2.3. Those factors and organizations are important to know about, but we were far more interested in a different question: Why do these companies have the power to inflate list prices at all? This power to affect prices is called market power, and in healthy capitalistic markets, manufacturers and distributors have very little to no market power; they simply have to price the product just above the cost of production and distribution to remain competitive, but still make a profit. In the US healthcare industry, however, manufacturers, distributors, and other groups seem to have an unhealthy and unnatural ability to drive up list prices. My team discovered that this “artificial market power” is apparently due to constraints and stimulation placed on the market by government and regulatory agencies, such as extreme barriers to entry, the plethora of market exclusivity programs provided to manufacturers by the FDA, legislation (like the BPCI Act), etc. This artificial market power cultivates “dysfunctional capitalism” where value is not spread across all parties evenly as in healthy capitalism, but instead corporations leverage their artificial market power and the patient’s need for the product to facilitate a backwards flow of value from the consumer to themselves.
For insulin specifically, you are all intimately aware of the devastating price gouging that has gotten steadily worse over the years. You may not know that a solution to the insulin crisis called the Biologics Price Competition and Innovation Act (the BPCI Act) of 2009 was proposed and set into effect over a decade ago. Unfortunately, this helpful and extremely necessary solution ultimately made the situation exponentially worse because of its execution. This legislation was intended to reduce the price of insulin and other drugs not by mandating a cap, but by allowing competing companies to more easily enter the market and produce generic insulin. Essentially, this would’ve set the market right by removing some of the artificial power of the three incumbent companies and, eventually, forcing all companies involved to compete using low prices and high quality. Instead, probably due to lobbying, this bill was altered to include a ten year delay before going into effect for insulin, which has ensured absolute market exclusivity for the three current manufacturers over the past decade. That artificially mandated exclusivity has given those companies and their supply chain partners the ability to drive insulin prices up significantly further than they were even in 2009 when this was already a crisis.
As for what this means moving forward, there’s good news, bad news, and worse news. The good news is that the ten year delay ended on March 23rd of last year (2020), so competition will very likely be entering the market before the end of this year (2021). Competition will certainly reduce prices, but the bad news is that it will probably take time. The worse news is that it’s unclear how much the price will drop even years from now. The BPCI Act only removed one aspect of the artificial market power present in the system; even after competition enters, the market will still be subject to the same constraints and stimulation that plague the rest of the healthcare system. So we’re very hopeful that the situation will improve dramatically, but similar issues will continue to hold the price of insulin and other medications higher than they should be.
So what does all of this mean for the PhyR Project? When we learned that our manufacturing technology couldn’t fix the problem, we hoped that our in-home device powered by the PhyR technology could. Unfortunately, that solution was met with even more regulatory challenges because it would be located in the home and the insulin produced would contain C-peptide (the main protein required to repair damage caused by T1D), which has never been done commercially before. Additionally, we would still need to engage with insurance companies and distributors after getting FDA approval, all of which would drive up the cost just like it has in the current system. In the end, we saw three terrible options: 1) start a company that would end up overcharging for insulin just like the current manufacturers, 2) license the technology to an established company so they can overcharge for insulin like the current manufacturers, or 3) walk away. As you can imagine, that was a very frustrating position to be in after working for so long and actually succeeding with the technology. Still, we couldn’t imagine how much more frustrating it must be for you, the ones subject to such an unjust medical system. That thought motivated us to completely eliminate all three of the above as options and begin searching for a new one.
I do not take the following lightly. Believe me when I say that my team searched for months on end and looked at the problems from every angle possible to us. We found many promising solutions, most involving drastic acts by congress or the use of millions or even billions of dollars, but we only found one solution that could possibly be achieved right now by a few college students. We do understand the gravity of our proposal. As you read, keep in mind the desperate situation that is the American healthcare system. From section 2.3 of the packet:
“With that reasoning and the entire preceding analysis in mind, we propose a radical solution to both the insulin crisis and the problems facing the United States healthcare system at large. Our analysis suggests that the high costs of medication are due to artificial market influences and a dysfunctional capitalistic system that result in the consumer often having only two options: Pay an artificially high price or die. Our solution is the introduction of a third option: Non-government regulated, low-cost medication. In this case, insulin.
“We acknowledge the importance of safety and efficacy regulations, especially for the therapeutic drug industry. We do not propose the abolishment of the current regulatory system; indeed, such a change would probably be impossible in the current system for the reasons discussed above. With those points in mind, we know we need a solution that does not depend on cooperation from either regulatory agencies (those introducing artificial market power) or current pharmaceutical manufacturing and distribution companies (those organizations exhibiting dysfunctional capitalistic behavior). However, we also know that it is illegal to distribute non-FDA approved medications. Finally and most importantly, we are aware of the absolute necessity to uphold efficacy and safety standards even without FDA approval.
“By virtue of our technology, we find ourselves in a unique position to offer a solution which fulfills all of the above issues: our point-of-care production device. Though we had previously proposed labelling it as a drug and/or medical device, we realized that a group with access to similar technology could make a simple change in marketing that would eliminate the need for FDA approval, namely removal of the claim that the insulin produced is injectable. As such, theoretically, the device could be labelled as “For research purposes only,” but include a guarantee that all insulin produced by it would be completely bioactive and have the exact same identity as natural human insulin (with stabilizing excipients matching those found in injectable formulations) or humanized insulin analogues (humanized by the addition of C-peptide). If a company had access to technology similar to ours and did this, they could potentially legally provide one or more of these devices and regular services to any group or individual who purchased them. Likewise, the group or individual purchasing the device could utilize it for whatever personal purposes they want or need to fulfill. This would provide a third option to consumers: Pay an enormous price, die, or purchase a research device and produce their own medication.
“We believe that the current United States healthcare system removes the basic human right to choose in some situations in favor of maintaining a dysfunctional capitalistic market. More specifically, we believe that each individual has a right to choose an alternative to the current options—poverty or death—even if that alternative presents some level of uncertainty and risk due to lack of government regulation. We also believe that the existence of another option may facilitate some rectification of the current dysfunctional capitalistic system with regard to the distribution of value. Finally, we are confident that the hypothetical solution proposed above would provide an adequate alternative option in addition to several other specific benefits, such as improved overall health and medical independence even in the face of a chronic illness.”
Obviously, we received very mixed reactions when we presented this at the University of Utah. One judge anonymously said this:
“I am completely bothered by the (lack of) ethics in this team. The team proposes to circumvent the huge cost of obtaining FDA approval by selling this equipment "for research purposes." However, their plan is actually to sell the equipment for individual diabetic patient production and use of insulin as a drug. The drug is being produced by a novel pathway, which absolutely requires FDA approval before patient use. I am appalled by the plans proposed here, even though I am completely sympathetic with the plight of diabetics and oligopolistic insulin pricing.”
Another judge said quite the opposite:
“I am "blown away" by this project, which represents True* Innovation both in the product itself and in the way this team views capitalism and the desperate need for it to evolve past its current dysfunctional state. I am sad that current COVID and other realities of our world and our state will not permit me to convey my admiration and respect for this team face to face. I hope this team receives all support that B2B can provide. *As opposed to what's often portrayed as 'innovation', but in reality is anything but that.”
It’s important for me to note that we have not pursued this further since the Bench to Bedside program in late September. We did not receive the support we needed from the University of Utah and the negative comments, while equal in mass (but not volume) to the positive comments, made us concerned that this radical solution may not be well accepted or even needed.
Moving forward, we want to publicly release all our research and the information necessary to replicate the PhyR technology and device so any individual can utilize it if they want (this release is in the form of the packet below). We are maintaining control of the IP to restrict large corporations from using it, but we want everyone else to have access to the technology if they can use it to help themselves or anyone else, so we are offering free licenses to anyone interested. If you have any questions about that, or if you need information that you can’t find in the system description in the packet (e.g. specific system pressure, reagent concentrations, media composition, etc.), please email me directly at connor.d.behr at gmail.com.
Due to uncertainty, I have disbanded my team for the time being. It's unclear if our solution would even be acceptable to those it's intended to help. I stand at your service with my limited resources, but I also plan to experiment with a variation of the PhyR technology in the chemical manufacturing industry to keep both my family and the technology afloat in the meantime. Again, please don't hesitate to reach out with any questions or comments; my email is above.
I cannot express the depth of my appreciation for you and your support through this journey. I hope this is not the last update I post to this campaign. If it is, I hope wholeheartedly that our work over the last two years has helped.
With deepest gratitude and sincerity,
Connor Behr and The PhyR Project Team
Link to the information packet: https://drive.google.com/file/d/1JJa8GycAJxXy800TC8qp3m7JekNeGvSb/view?usp=sharing
( All other updates are now at the bottom of this page because that makes more sense) :)
Hi, I’m Connor Behr. If you are or know someone who is diabetic, you may know of the current insulin crisis in the United States and other countries. There are currently only three insulin manufacturers serving the U.S. market: Eli Lilly, Novo Nordisk, and Sanofi. These suppliers are struggling to keep up with the increasing demand from the growing number of type 1 and type 2 diabetes cases.
According to an article published by the American Diabetes Association, there were 30.3 million Americans, 9.4% of the population, with diabetes in 2015. 1.5 million more people are diagnosed every year in America alone. As the three insulin manufacturers attempt to supply these demands, insurance and insulin prices steadily go up. Those who can't pay the increasing rates often need to turn to friends and family for financial aid in order to get what they need to survive. A quick search on GoFundMe for fundraisers with the word "insulin" yields nearly 6,500 results, most of which are raising money for insulin and insulin pumps. Still worse, a study conducted by Yale last year found that 25% of diabetic patients were using less insulin than is healthy in order to save money.
I learned of the insulin crisis late last year as my family discussed it while my wife and I were visiting. My sister is type 1 diabetic, so this topic is close to home. When I did more research and the seriousness of the issue became more clear, I decided to apply my skills and try for a solution. I am currently a student at BYU Pathways in pursuit of a degree in molecular biology, which I have loved and studied since I was about 12 years old. I am skilled in the field and have experience shadowing at ARUP Laboratories and a summer internship at the Huntsman Cancer Institute. I received the Emperor Science Award in 2017 for my research in cancer development and treatment and I have conducted related gene and protein expression projects on my own.
After months of hard work, I have completed plans for a method of synthetic insulin production that, if it works, will decrease the cost of insulin by several times and increase production speed and efficiency. We call it the PhyR Project, short for Physarum Reactor. With proper funding for testing and development, this patent-pending method will hopefully reduce insulin costs and insurance costs across the board and create a production rate rapid enough to meet demands with ease. Additionally, this production technique is an almost exact duplicate of the method used by the human body and the resulting insulin solution should be indistinguishable from the insulin solution secreted by our pancreatic beta cells. This means that some symptoms and health problems that diabetics experience may be reversed and they will have more control over their blood sugar levels (this will be discussed in the science section). Finally, this method is intended to eventually make it possible for patients to produce insulin in their own homes.
I have been putting time and resources into this project for months, but with my wife and I both being in school, money is tight and funds for this project are slow to come. I want to work on this problem full-time in hopes of finding a solution as soon as possible for my sister and all others suffering, so if we meet our goal, that's exactly where the funds will go. If the goal is not met, I will still work as much as I can on the project and finish it as soon as I can, but I'm afraid it will take much longer. That's why I came here. I will outline the entire project below, answer any questions, and keep everyone updated so you know what your contributions are doing and how you're helping. Please help if you can; we are very grateful for any donations. Remember, we're trying to help people spend less money and be more healthy, so please don't go out of your budget to donate; we recommend donations anywhere from $1 to $100. And if your budget doesn't allow it, sharing this page is the next best way to contribute and is also greatly appreciated. Thank you so much for reading!
The Science:
In this section, I will explain how the new insulin production method works. This method is patent pending, therefore I can safely disclose all information about it and answer any questions, so please ask. Since this is a pretty scientifically comprehensive description, it might be difficult to understand. Again, please ask questions. You can comment or contact me directly at connor.d.behr at gmail.com. We are also working on a video that should be a little less like trudging through honey, so that will be coming soon.
First of all, it’s important to understand how insulin is naturally produced in the human body. Insulin comes from the pancreas and is produced by specialized cells called beta cells. These cells first transcribe the insulin gene into RNA, which travels out of the nucleus and to something called the endoplasmic reticulum, which is where some proteins are processed and sent to other places in the cell. Here, the RNA is translated into a protein that’s called preproinsulin, but at this stage the insulin is immature and unusable, so it needs to be processed. The preproinsulin is put into the endoplasmic reticulum and folded, then the beginning of the sequence is trimmed off to produce proinsulin. The proinsulin then gets sent to another place in the cell called the golgi apparatus, which is the part of the cell that secretes proteins (sends them outside the cell). Here, the proinsulin is loaded into storage bubbles called secretory vesicles.
At the same time as this is happening to the insulin, the cell is also producing three other proteins named PC1, PC2, and CPE. These proteins take the same route as the insulin, so the golgi apparatus puts them into the secretory vesicles (the storage bubbles) with the proinsulin. Here, the PC1, PC2, and CPE proteins cut and trim the proinsulin into three different pieces, called the A chain, the C-peptide, and the B chain. The A chain and B chain were folded over each other and bound together back when the protein got processed in the endoplasmic reticulum, so those pieces stay together. That’s what mature, usable insulin is--the A and B chains linked together in the middle, but snipped on the ends.
This image from Wikipedia is a good visual for the process we’re talking about:

So the insulin protein you get looks like this (image credit: OpenStax Biology ):

Now that the insulin is mature and active, it is ready to be used. When the beta cells detect high blood glucose levels, they release the secretory vesicles filled with insulin, the C-peptide, PC1, PC2, and CPE into the blood. The insulin goes on to enable other cells in the body to absorb and use the sugar in the blood, but we don’t really know if the other four proteins have functions. Some scientists have theorized that these proteins do have other purposes in the body, especially C-peptide. This article published by Oxford Academic discusses strong evidence that C-peptide replacement therapy may prevent the complications related to blood vessels that many diabetics experience, such as vascular inflammation, endothelial cell death, and neointima formation. The authors also go on to discuss organ-specific complications that may be treated by C-peptide supplementation, such as retinopathy (damage to eyes), nephropathy (issues with kidney function), neuropathy (nerve damage and pain), impaired wound healing, and inflammation. The paper also mentions improved blood sugar control and stability when insulin and C-peptide are used together. There are strong indications that C-peptide and the other proteins secreted alongside insulin may have other functions in the body, which would explain some of the symptoms that diabetic patients experience, especially type 1. Just like how high blood sugar is caused by the pancreas not producing insulin in type 1 diabetes, other symptoms of diabetes may be caused by the pancreas not producing the other four proteins.
Now that you understand how insulin is naturally produced, let’s briefly cover how synthetic insulin is produced. First, the DNA sequence of the A chain and B chain of insulin are separated and inserted into bacteria or yeast because they are easy to grow. These bacteria or yeast are then put into large metal containers called bioreactors or fermentors. These bioreactors contain media (food) for the microorganisms and systems that maintain the temperature, oxygenation, and movement of the liquid at optimum levels. As the bacteria or yeast grow, they transcribe and translate the DNA put into them encoding the A chain and B chain of insulin. These proteins are produced separately and the bacteria cannot bind them together because they lack an endoplasmic reticulum (yeast do have endoplasmic reticuli, but the binding step is still done by the scientists as part of purification). Once the bacteria or yeast have grown as much as they can in the bioreactor, the tank is drained and the microorganisms are split open, spilling their contents, including the A and B chains (this requires each consecutive batch to be restarted and regrown). After that, the A and B chains are purified out of the liquid, then are chemically treated to bind them together in the middle. Finally, the completed insulin is purified once again, quality tested, and put into glass bottles that are then sold to customers and distributors.
Alright, first of all congratulations on getting this far, four paragraphs of raw science is quite the feat! Give yourself a pat on the back, take five, get a snack, do you.
Now, let’s get to the good stuff. The project we’re proposing uses an organism called Physarum polycephalum to produce insulin in the same way as the human body does. Physarum polycephalum is critical to this method because of its unique properties. It is a multinucleated single celled organism, which means that it’s one massive cell with millions of nuclei. It’s biologically immortal (which means it won’t die of old age) and will continue to grow exponentially as long as it has room, food, and water. It is also eukaryotic, which means it has endoplasmic reticuli (lots, actually). Most importantly of all, however, is that Physarum’s ability to grow so quickly is due to its amount of nuclei, ribosomes (proteins in the cell that translate RNA into protein), and other cellular machinery. It is literally a protein producing machine. As a result, it’s the perfect candidate to produce synthetic insulin quickly and efficiently.
To achieve this, Physarum polycephalum will be modified with DNA encoding human preproinsulin and PC1, PC2, and CPE to process it into insulin with the same method as the human body. Each of the millions of Physarum nuclei will mass produce preproinsulin RNA which will be transported to the millions of endoplasmic reticuli where they will be trimmed, folded and bound, then transported to the millions of golgi apparatus where they will be packaged into secretory vesicles along with PC1, PC2, and CPE where they will be processed into mature insulin proteins, then secreted as insulin, C-peptide, PC1, PC2, and CPE, just as in the human body, but on a much larger, faster, more efficient scale.
Physarum polycephalum specimens that have been modified to do this will be grown normally and rinsed occasionally to harvest the secreted proteins, then the solution will be purified and should be ready for use.
Using this method will reduce the time, energy, cost, and materials required to produce insulin. It does so by eliminating a purification step and the chemical binding step necessary in other techniques, as well as producing the proteins in a more efficient organism system to begin with. This system also does not require every batch to be started from scratch, but instead is continuously producing as needed. This method will also produce the other proteins naturally released in the human body alongside insulin, which may help treat other symptoms of diabetes as well.
Great job, you made it! Thanks again for reading and please ask any questions you have!
Also, please share this if you like what we're trying to do! :)
Update - 04/17/2019
Thank you so much to everyone who has donated already! The PhyR Project made the front page of the Daily Herald today; click here to see the article. More to come!
Update - 04/19/2019
Thank you all for the continuing support! Check out the latest article on ABC 4 News that aired last night and was published this morning! We still have a long way to go, but we can do it!
Update - 05/23/2019
Just a quick update for those of you who are interested or seeing this for the first time: Thank you to everyone who has donated! You've allowed me to begin modifying Physarum and start building the PhyR machine! I'm still waiting on some samples to get shipped (I want to show you all the reagents and samples so you know how your contributions are helping), but we are less than a few weeks out from that. As you know, we still have a long way to go, but I'm confident that we can do this!
Also, thank you to everyone who came to the T1 International meetings in Salt Lake City and Brigham City this month. I was shocked by your support and hope for this project. We always knew we were out to save lives, but it's more real when those lives start cheering you on.
More coming soon! Thank you!!!
Update - 06/10/2019
Big news! I've been chosen to speak at TEDxSaltLakeCity about the insulin crisis and the PhyR Project! The event will be on September 21st at Kingsbury Hall in Salt Lake. We'd love to see you there! Check it out: https://www.tedxsaltlakecity.com/
As usual, thank you so much for all the support!
Update - 09/23/2019
TEDxSaltLakeCity was amazing! I'm overwhelmed by the support and love from all of you! It was a wonderful opportunity, and I'm truly in awe of the people I got to work with. So much good!
I was really hoping that the video of the talk would be ready in a few days, but it sounds like it might take a few months for them to release it... I'm excited for everyone who wasn't there to be able to see it as soon as possible though!
The comments section made me cry earlier today. I'm so grateful for your hope and faith in me and in this project. I cannot thank you all enough.
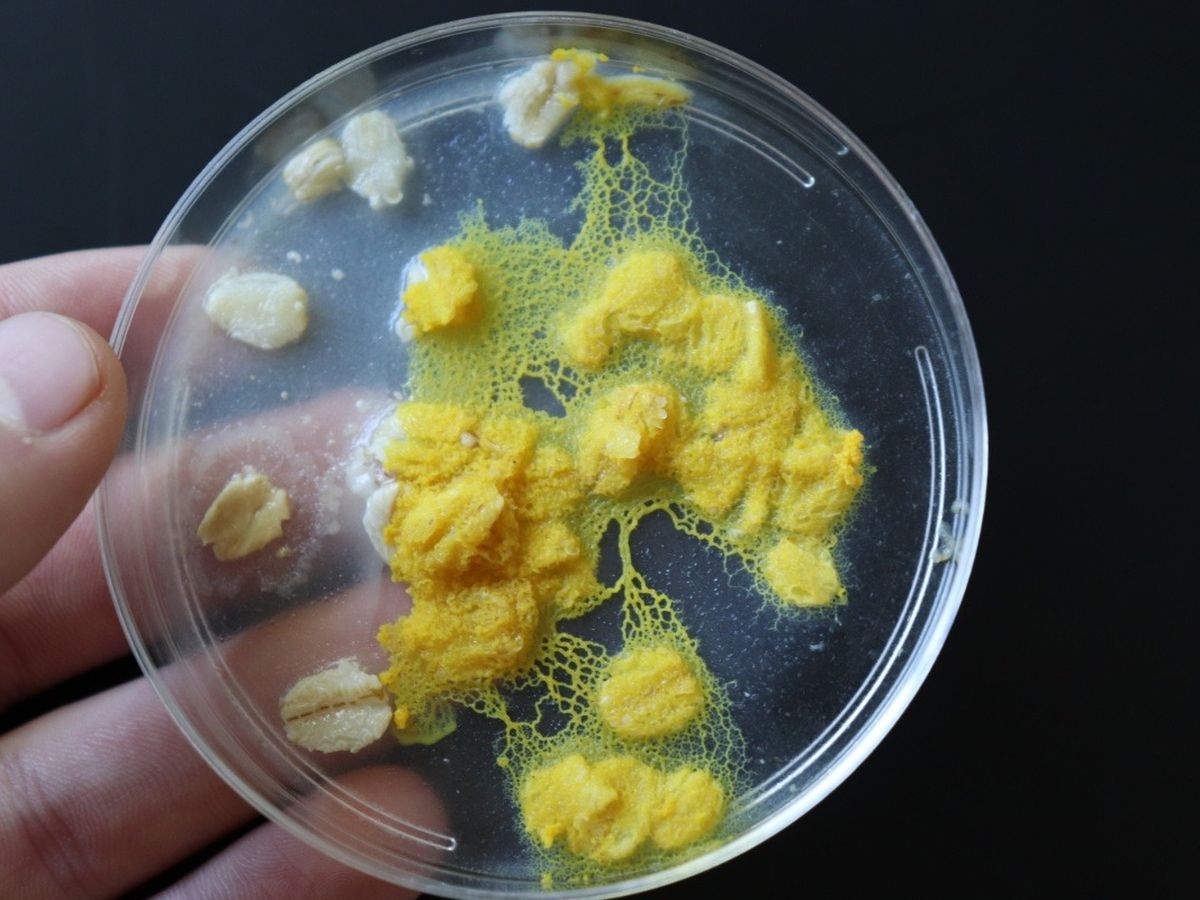
Your donations are invaluable! So much progress has been made! Anyone who has seen my talk may recognize this image:

I'm so happy to tell you guys that this Physarum specimen is now producing some form of insulin!!! I'm in the process of running a lot more tests to see if the insulin is fully developed or only partially, but I'll keep you posted! Essentially, we know it's expressing the DNA I put into it, so now we just need to find out if it's making the proteins exactly the right way, or if I need to do a little more fine tuning.
These last few months have had several major breakthroughs and we're now this much closer to creating a specimen that produces perfect insulin, then assembling the first PhyR machine prototype! Obviously so much more work has to be done and many more resources are needed, but we're doing it! Again, I cannot thank you guys enough for the donations, shares, support, love, and faith! This is possible only because of you and everything you do. I can't do this alone. So thank you thank you thank you!!! More updates to come! As always, if any of you have questions or want to hear the details, I'm always so down to talk about any of this.
Update - 06/17/20
We're still alive! Since last fall, the PhyR Project has won various awards in competitions at Brigham Young University, and Physarum and I were featured in an article the Utah Business Magazine!
Read the article here: https://www.utahbusiness.com/affordable-insulin-crisis/
We've kept up progress in our protein expression as well, and are several iterations along in the prototyping of the PhyR machine. Thank you all so much--this is only possible because of your continued support! More soon, and as always, feel free to reach out!
The full update is long, so here is a bullet point summary:
~ We have successfully produced functional insulin and C-peptide using Physarum polycephalum. We have also designed a device that, with more development, could allow people to produce their own safe, functional insulin at home.
~ We have discovered that dysfunction in the traditional medication commercialization process and market structure is to blame for high medication prices, especially insulin.
~ Introducing our PhyR technology to the market via the traditional route would have little to no effect on insulin prices because manufacturing costs are not the problem. Instead, we have created a new and somewhat radical solution that is achievable by individuals or a small group with few resources and could produce effective, very low cost insulin.
~ We are hesitant to proceed with the new solution because it's unclear if people want it. For that reason, we have published our work, technology, and plans here (in the information packet link below) so anyone who wants to use it can, and so we can get feedback from you.
~ Please message or email me feedback on our research and technology; we would like to hear your opinions on if the solution we've proposed could be helpful and viable.
Dear supporters,
Thank you so much for your continuing generosity and encouragement. You’ve helped sustain this project for almost two years even in the midst of struggles brought on by a global pandemic. I cannot express how grateful I am for you and how I respect your strength.
During these last two years, I and several volunteers and team members have put your donations to work in developing the theoretical technology I described in April of 2019. We have had the opportunity to present the technology many times across the country and even internationally, including events at Brigham Young University, the University of Utah, the National University of Singapore, and with many groups of individual supporters. It seems like we’re met with almost overwhelming encouragement and support everywhere we mention the goals of this project. Nearly everyone I’ve personally encountered believes that medication and care are too expensive and that change is necessary in the American healthcare system, especially for those with diabetes.
The goal of The PhyR Project has always been to develop innovative technology with the intention of driving down the cost of insulin and providing the other necessary proteins that current insulin lacks. I am ecstatic to tell you that we have successfully produced insulin and those other proteins using Physarum polycephalum and we have designed a system (the PhyR machine) capable of capturing, purifying, and preparing that insulin for use in the human body to treat type 1 and type 2 diabetes. Even more excitingly, the system we have designed could potentially be used by an individual to produce their own insulin at home, affordably, and independent of insurance and pharmaceutical companies. This system requires more extensive development before it can be trusted to produce insulin safely and reliably at home, but we have proven that it is possible.
While developing this technology, we also dedicated significant time and resources to determining what has gone wrong in the US healthcare system to allow insulin and other medications to cost so much. We found what we believe is the answer and published our analysis in our presentation packet for the University of Utah’s Bench to Bedside program. The packet is included below for public access and I encourage anyone interested to read it carefully; it contains the sum of our work and research over the last two years. In short, we discovered that manufacturing costs have little to no relation to insulin list prices. From section 2.2.3 of the packet:
“...assume a technological advancement reduced the cost of producing, purifying, validating, and packaging insulin by half ($15 per vial). Implementation of that advancement would reduce the list price of insulin from $275 per vial to $260 per vial. If a similar advancement reduced the manufacturing cost to a tenth of the original ($3 per vial), it would lower the list price to $248. If it was reduced to one hundredth of the original cost ($0.3 per vial), the price would be lowered to $245.75. Finally, if it was somehow possible to produce, purify, validate, and package insulin with no manufacturing costs whatsoever, the consumer would still pay $245.45 for a single vial of insulin in the current system. As the system currently exists, no degree of innovation will have any power to significantly reduce the cost of insulin or most other therapeutic drugs because manufacturing cost contributes so little to the actual price the consumer pays.”
As you know, the PhyR technology we’ve been developing is a manufacturing innovation that could significantly reduce the cost of making insulin. Unfortunately, as the above excerpt details, we cannot significantly affect the list price of insulin through manufacturing innovation because the price is being driven up by other factors.
Factors and companies that directly inflate the list price of insulin are discussed in detail in section 2.2.3. Those factors and organizations are important to know about, but we were far more interested in a different question: Why do these companies have the power to inflate list prices at all? This power to affect prices is called market power, and in healthy capitalistic markets, manufacturers and distributors have very little to no market power; they simply have to price the product just above the cost of production and distribution to remain competitive, but still make a profit. In the US healthcare industry, however, manufacturers, distributors, and other groups seem to have an unhealthy and unnatural ability to drive up list prices. My team discovered that this “artificial market power” is apparently due to constraints and stimulation placed on the market by government and regulatory agencies, such as extreme barriers to entry, the plethora of market exclusivity programs provided to manufacturers by the FDA, legislation (like the BPCI Act), etc. This artificial market power cultivates “dysfunctional capitalism” where value is not spread across all parties evenly as in healthy capitalism, but instead corporations leverage their artificial market power and the patient’s need for the product to facilitate a backwards flow of value from the consumer to themselves.
For insulin specifically, you are all intimately aware of the devastating price gouging that has gotten steadily worse over the years. You may not know that a solution to the insulin crisis called the Biologics Price Competition and Innovation Act (the BPCI Act) of 2009 was proposed and set into effect over a decade ago. Unfortunately, this helpful and extremely necessary solution ultimately made the situation exponentially worse because of its execution. This legislation was intended to reduce the price of insulin and other drugs not by mandating a cap, but by allowing competing companies to more easily enter the market and produce generic insulin. Essentially, this would’ve set the market right by removing some of the artificial power of the three incumbent companies and, eventually, forcing all companies involved to compete using low prices and high quality. Instead, probably due to lobbying, this bill was altered to include a ten year delay before going into effect for insulin, which has ensured absolute market exclusivity for the three current manufacturers over the past decade. That artificially mandated exclusivity has given those companies and their supply chain partners the ability to drive insulin prices up significantly further than they were even in 2009 when this was already a crisis.
As for what this means moving forward, there’s good news, bad news, and worse news. The good news is that the ten year delay ended on March 23rd of last year (2020), so competition will very likely be entering the market before the end of this year (2021). Competition will certainly reduce prices, but the bad news is that it will probably take time. The worse news is that it’s unclear how much the price will drop even years from now. The BPCI Act only removed one aspect of the artificial market power present in the system; even after competition enters, the market will still be subject to the same constraints and stimulation that plague the rest of the healthcare system. So we’re very hopeful that the situation will improve dramatically, but similar issues will continue to hold the price of insulin and other medications higher than they should be.
So what does all of this mean for the PhyR Project? When we learned that our manufacturing technology couldn’t fix the problem, we hoped that our in-home device powered by the PhyR technology could. Unfortunately, that solution was met with even more regulatory challenges because it would be located in the home and the insulin produced would contain C-peptide (the main protein required to repair damage caused by T1D), which has never been done commercially before. Additionally, we would still need to engage with insurance companies and distributors after getting FDA approval, all of which would drive up the cost just like it has in the current system. In the end, we saw three terrible options: 1) start a company that would end up overcharging for insulin just like the current manufacturers, 2) license the technology to an established company so they can overcharge for insulin like the current manufacturers, or 3) walk away. As you can imagine, that was a very frustrating position to be in after working for so long and actually succeeding with the technology. Still, we couldn’t imagine how much more frustrating it must be for you, the ones subject to such an unjust medical system. That thought motivated us to completely eliminate all three of the above as options and begin searching for a new one.
I do not take the following lightly. Believe me when I say that my team searched for months on end and looked at the problems from every angle possible to us. We found many promising solutions, most involving drastic acts by congress or the use of millions or even billions of dollars, but we only found one solution that could possibly be achieved right now by a few college students. We do understand the gravity of our proposal. As you read, keep in mind the desperate situation that is the American healthcare system. From section 2.3 of the packet:
“With that reasoning and the entire preceding analysis in mind, we propose a radical solution to both the insulin crisis and the problems facing the United States healthcare system at large. Our analysis suggests that the high costs of medication are due to artificial market influences and a dysfunctional capitalistic system that result in the consumer often having only two options: Pay an artificially high price or die. Our solution is the introduction of a third option: Non-government regulated, low-cost medication. In this case, insulin.
“We acknowledge the importance of safety and efficacy regulations, especially for the therapeutic drug industry. We do not propose the abolishment of the current regulatory system; indeed, such a change would probably be impossible in the current system for the reasons discussed above. With those points in mind, we know we need a solution that does not depend on cooperation from either regulatory agencies (those introducing artificial market power) or current pharmaceutical manufacturing and distribution companies (those organizations exhibiting dysfunctional capitalistic behavior). However, we also know that it is illegal to distribute non-FDA approved medications. Finally and most importantly, we are aware of the absolute necessity to uphold efficacy and safety standards even without FDA approval.
“By virtue of our technology, we find ourselves in a unique position to offer a solution which fulfills all of the above issues: our point-of-care production device. Though we had previously proposed labelling it as a drug and/or medical device, we realized that a group with access to similar technology could make a simple change in marketing that would eliminate the need for FDA approval, namely removal of the claim that the insulin produced is injectable. As such, theoretically, the device could be labelled as “For research purposes only,” but include a guarantee that all insulin produced by it would be completely bioactive and have the exact same identity as natural human insulin (with stabilizing excipients matching those found in injectable formulations) or humanized insulin analogues (humanized by the addition of C-peptide). If a company had access to technology similar to ours and did this, they could potentially legally provide one or more of these devices and regular services to any group or individual who purchased them. Likewise, the group or individual purchasing the device could utilize it for whatever personal purposes they want or need to fulfill. This would provide a third option to consumers: Pay an enormous price, die, or purchase a research device and produce their own medication.
“We believe that the current United States healthcare system removes the basic human right to choose in some situations in favor of maintaining a dysfunctional capitalistic market. More specifically, we believe that each individual has a right to choose an alternative to the current options—poverty or death—even if that alternative presents some level of uncertainty and risk due to lack of government regulation. We also believe that the existence of another option may facilitate some rectification of the current dysfunctional capitalistic system with regard to the distribution of value. Finally, we are confident that the hypothetical solution proposed above would provide an adequate alternative option in addition to several other specific benefits, such as improved overall health and medical independence even in the face of a chronic illness.”
Obviously, we received very mixed reactions when we presented this at the University of Utah. One judge anonymously said this:
“I am completely bothered by the (lack of) ethics in this team. The team proposes to circumvent the huge cost of obtaining FDA approval by selling this equipment "for research purposes." However, their plan is actually to sell the equipment for individual diabetic patient production and use of insulin as a drug. The drug is being produced by a novel pathway, which absolutely requires FDA approval before patient use. I am appalled by the plans proposed here, even though I am completely sympathetic with the plight of diabetics and oligopolistic insulin pricing.”
Another judge said quite the opposite:
“I am "blown away" by this project, which represents True* Innovation both in the product itself and in the way this team views capitalism and the desperate need for it to evolve past its current dysfunctional state. I am sad that current COVID and other realities of our world and our state will not permit me to convey my admiration and respect for this team face to face. I hope this team receives all support that B2B can provide. *As opposed to what's often portrayed as 'innovation', but in reality is anything but that.”
It’s important for me to note that we have not pursued this further since the Bench to Bedside program in late September. We did not receive the support we needed from the University of Utah and the negative comments, while equal in mass (but not volume) to the positive comments, made us concerned that this radical solution may not be well accepted or even needed.
Moving forward, we want to publicly release all our research and the information necessary to replicate the PhyR technology and device so any individual can utilize it if they want (this release is in the form of the packet below). We are maintaining control of the IP to restrict large corporations from using it, but we want everyone else to have access to the technology if they can use it to help themselves or anyone else, so we are offering free licenses to anyone interested. If you have any questions about that, or if you need information that you can’t find in the system description in the packet (e.g. specific system pressure, reagent concentrations, media composition, etc.), please email me directly at connor.d.behr at gmail.com.
Due to uncertainty, I have disbanded my team for the time being. It's unclear if our solution would even be acceptable to those it's intended to help. I stand at your service with my limited resources, but I also plan to experiment with a variation of the PhyR technology in the chemical manufacturing industry to keep both my family and the technology afloat in the meantime. Again, please don't hesitate to reach out with any questions or comments; my email is above.
I cannot express the depth of my appreciation for you and your support through this journey. I hope this is not the last update I post to this campaign. If it is, I hope wholeheartedly that our work over the last two years has helped.
With deepest gratitude and sincerity,
Connor Behr and The PhyR Project Team
Link to the information packet: https://drive.google.com/file/d/1JJa8GycAJxXy800TC8qp3m7JekNeGvSb/view?usp=sharing
( All other updates are now at the bottom of this page because that makes more sense) :)
Hi, I’m Connor Behr. If you are or know someone who is diabetic, you may know of the current insulin crisis in the United States and other countries. There are currently only three insulin manufacturers serving the U.S. market: Eli Lilly, Novo Nordisk, and Sanofi. These suppliers are struggling to keep up with the increasing demand from the growing number of type 1 and type 2 diabetes cases.
According to an article published by the American Diabetes Association, there were 30.3 million Americans, 9.4% of the population, with diabetes in 2015. 1.5 million more people are diagnosed every year in America alone. As the three insulin manufacturers attempt to supply these demands, insurance and insulin prices steadily go up. Those who can't pay the increasing rates often need to turn to friends and family for financial aid in order to get what they need to survive. A quick search on GoFundMe for fundraisers with the word "insulin" yields nearly 6,500 results, most of which are raising money for insulin and insulin pumps. Still worse, a study conducted by Yale last year found that 25% of diabetic patients were using less insulin than is healthy in order to save money.
I learned of the insulin crisis late last year as my family discussed it while my wife and I were visiting. My sister is type 1 diabetic, so this topic is close to home. When I did more research and the seriousness of the issue became more clear, I decided to apply my skills and try for a solution. I am currently a student at BYU Pathways in pursuit of a degree in molecular biology, which I have loved and studied since I was about 12 years old. I am skilled in the field and have experience shadowing at ARUP Laboratories and a summer internship at the Huntsman Cancer Institute. I received the Emperor Science Award in 2017 for my research in cancer development and treatment and I have conducted related gene and protein expression projects on my own.
After months of hard work, I have completed plans for a method of synthetic insulin production that, if it works, will decrease the cost of insulin by several times and increase production speed and efficiency. We call it the PhyR Project, short for Physarum Reactor. With proper funding for testing and development, this patent-pending method will hopefully reduce insulin costs and insurance costs across the board and create a production rate rapid enough to meet demands with ease. Additionally, this production technique is an almost exact duplicate of the method used by the human body and the resulting insulin solution should be indistinguishable from the insulin solution secreted by our pancreatic beta cells. This means that some symptoms and health problems that diabetics experience may be reversed and they will have more control over their blood sugar levels (this will be discussed in the science section). Finally, this method is intended to eventually make it possible for patients to produce insulin in their own homes.
I have been putting time and resources into this project for months, but with my wife and I both being in school, money is tight and funds for this project are slow to come. I want to work on this problem full-time in hopes of finding a solution as soon as possible for my sister and all others suffering, so if we meet our goal, that's exactly where the funds will go. If the goal is not met, I will still work as much as I can on the project and finish it as soon as I can, but I'm afraid it will take much longer. That's why I came here. I will outline the entire project below, answer any questions, and keep everyone updated so you know what your contributions are doing and how you're helping. Please help if you can; we are very grateful for any donations. Remember, we're trying to help people spend less money and be more healthy, so please don't go out of your budget to donate; we recommend donations anywhere from $1 to $100. And if your budget doesn't allow it, sharing this page is the next best way to contribute and is also greatly appreciated. Thank you so much for reading!
The Science:
In this section, I will explain how the new insulin production method works. This method is patent pending, therefore I can safely disclose all information about it and answer any questions, so please ask. Since this is a pretty scientifically comprehensive description, it might be difficult to understand. Again, please ask questions. You can comment or contact me directly at connor.d.behr at gmail.com. We are also working on a video that should be a little less like trudging through honey, so that will be coming soon.
First of all, it’s important to understand how insulin is naturally produced in the human body. Insulin comes from the pancreas and is produced by specialized cells called beta cells. These cells first transcribe the insulin gene into RNA, which travels out of the nucleus and to something called the endoplasmic reticulum, which is where some proteins are processed and sent to other places in the cell. Here, the RNA is translated into a protein that’s called preproinsulin, but at this stage the insulin is immature and unusable, so it needs to be processed. The preproinsulin is put into the endoplasmic reticulum and folded, then the beginning of the sequence is trimmed off to produce proinsulin. The proinsulin then gets sent to another place in the cell called the golgi apparatus, which is the part of the cell that secretes proteins (sends them outside the cell). Here, the proinsulin is loaded into storage bubbles called secretory vesicles.
At the same time as this is happening to the insulin, the cell is also producing three other proteins named PC1, PC2, and CPE. These proteins take the same route as the insulin, so the golgi apparatus puts them into the secretory vesicles (the storage bubbles) with the proinsulin. Here, the PC1, PC2, and CPE proteins cut and trim the proinsulin into three different pieces, called the A chain, the C-peptide, and the B chain. The A chain and B chain were folded over each other and bound together back when the protein got processed in the endoplasmic reticulum, so those pieces stay together. That’s what mature, usable insulin is--the A and B chains linked together in the middle, but snipped on the ends.
This image from Wikipedia is a good visual for the process we’re talking about:

So the insulin protein you get looks like this (image credit: OpenStax Biology ):

Now that the insulin is mature and active, it is ready to be used. When the beta cells detect high blood glucose levels, they release the secretory vesicles filled with insulin, the C-peptide, PC1, PC2, and CPE into the blood. The insulin goes on to enable other cells in the body to absorb and use the sugar in the blood, but we don’t really know if the other four proteins have functions. Some scientists have theorized that these proteins do have other purposes in the body, especially C-peptide. This article published by Oxford Academic discusses strong evidence that C-peptide replacement therapy may prevent the complications related to blood vessels that many diabetics experience, such as vascular inflammation, endothelial cell death, and neointima formation. The authors also go on to discuss organ-specific complications that may be treated by C-peptide supplementation, such as retinopathy (damage to eyes), nephropathy (issues with kidney function), neuropathy (nerve damage and pain), impaired wound healing, and inflammation. The paper also mentions improved blood sugar control and stability when insulin and C-peptide are used together. There are strong indications that C-peptide and the other proteins secreted alongside insulin may have other functions in the body, which would explain some of the symptoms that diabetic patients experience, especially type 1. Just like how high blood sugar is caused by the pancreas not producing insulin in type 1 diabetes, other symptoms of diabetes may be caused by the pancreas not producing the other four proteins.
Now that you understand how insulin is naturally produced, let’s briefly cover how synthetic insulin is produced. First, the DNA sequence of the A chain and B chain of insulin are separated and inserted into bacteria or yeast because they are easy to grow. These bacteria or yeast are then put into large metal containers called bioreactors or fermentors. These bioreactors contain media (food) for the microorganisms and systems that maintain the temperature, oxygenation, and movement of the liquid at optimum levels. As the bacteria or yeast grow, they transcribe and translate the DNA put into them encoding the A chain and B chain of insulin. These proteins are produced separately and the bacteria cannot bind them together because they lack an endoplasmic reticulum (yeast do have endoplasmic reticuli, but the binding step is still done by the scientists as part of purification). Once the bacteria or yeast have grown as much as they can in the bioreactor, the tank is drained and the microorganisms are split open, spilling their contents, including the A and B chains (this requires each consecutive batch to be restarted and regrown). After that, the A and B chains are purified out of the liquid, then are chemically treated to bind them together in the middle. Finally, the completed insulin is purified once again, quality tested, and put into glass bottles that are then sold to customers and distributors.
Alright, first of all congratulations on getting this far, four paragraphs of raw science is quite the feat! Give yourself a pat on the back, take five, get a snack, do you.
Now, let’s get to the good stuff. The project we’re proposing uses an organism called Physarum polycephalum to produce insulin in the same way as the human body does. Physarum polycephalum is critical to this method because of its unique properties. It is a multinucleated single celled organism, which means that it’s one massive cell with millions of nuclei. It’s biologically immortal (which means it won’t die of old age) and will continue to grow exponentially as long as it has room, food, and water. It is also eukaryotic, which means it has endoplasmic reticuli (lots, actually). Most importantly of all, however, is that Physarum’s ability to grow so quickly is due to its amount of nuclei, ribosomes (proteins in the cell that translate RNA into protein), and other cellular machinery. It is literally a protein producing machine. As a result, it’s the perfect candidate to produce synthetic insulin quickly and efficiently.
To achieve this, Physarum polycephalum will be modified with DNA encoding human preproinsulin and PC1, PC2, and CPE to process it into insulin with the same method as the human body. Each of the millions of Physarum nuclei will mass produce preproinsulin RNA which will be transported to the millions of endoplasmic reticuli where they will be trimmed, folded and bound, then transported to the millions of golgi apparatus where they will be packaged into secretory vesicles along with PC1, PC2, and CPE where they will be processed into mature insulin proteins, then secreted as insulin, C-peptide, PC1, PC2, and CPE, just as in the human body, but on a much larger, faster, more efficient scale.
Physarum polycephalum specimens that have been modified to do this will be grown normally and rinsed occasionally to harvest the secreted proteins, then the solution will be purified and should be ready for use.
Using this method will reduce the time, energy, cost, and materials required to produce insulin. It does so by eliminating a purification step and the chemical binding step necessary in other techniques, as well as producing the proteins in a more efficient organism system to begin with. This system also does not require every batch to be started from scratch, but instead is continuously producing as needed. This method will also produce the other proteins naturally released in the human body alongside insulin, which may help treat other symptoms of diabetes as well.
Great job, you made it! Thanks again for reading and please ask any questions you have!
Also, please share this if you like what we're trying to do! :)
Update - 04/17/2019
Thank you so much to everyone who has donated already! The PhyR Project made the front page of the Daily Herald today; click here to see the article. More to come!
Update - 04/19/2019
Thank you all for the continuing support! Check out the latest article on ABC 4 News that aired last night and was published this morning! We still have a long way to go, but we can do it!
Update - 05/23/2019
Just a quick update for those of you who are interested or seeing this for the first time: Thank you to everyone who has donated! You've allowed me to begin modifying Physarum and start building the PhyR machine! I'm still waiting on some samples to get shipped (I want to show you all the reagents and samples so you know how your contributions are helping), but we are less than a few weeks out from that. As you know, we still have a long way to go, but I'm confident that we can do this!
Also, thank you to everyone who came to the T1 International meetings in Salt Lake City and Brigham City this month. I was shocked by your support and hope for this project. We always knew we were out to save lives, but it's more real when those lives start cheering you on.
More coming soon! Thank you!!!
Update - 06/10/2019
Big news! I've been chosen to speak at TEDxSaltLakeCity about the insulin crisis and the PhyR Project! The event will be on September 21st at Kingsbury Hall in Salt Lake. We'd love to see you there! Check it out: https://www.tedxsaltlakecity.com/
As usual, thank you so much for all the support!
Update - 09/23/2019
TEDxSaltLakeCity was amazing! I'm overwhelmed by the support and love from all of you! It was a wonderful opportunity, and I'm truly in awe of the people I got to work with. So much good!
I was really hoping that the video of the talk would be ready in a few days, but it sounds like it might take a few months for them to release it... I'm excited for everyone who wasn't there to be able to see it as soon as possible though!
The comments section made me cry earlier today. I'm so grateful for your hope and faith in me and in this project. I cannot thank you all enough.
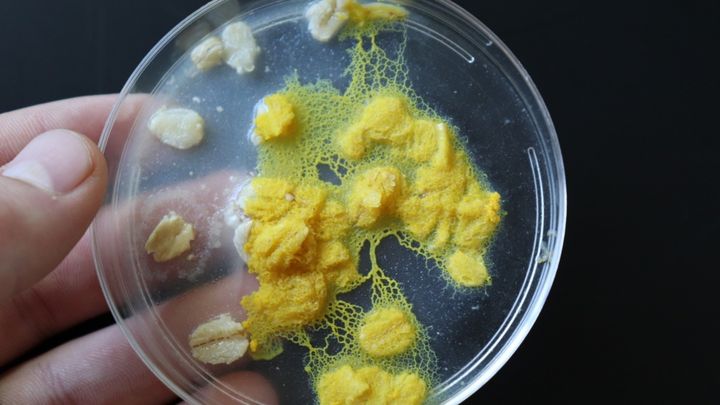
Your donations are invaluable! So much progress has been made! Anyone who has seen my talk may recognize this image:

I'm so happy to tell you guys that this Physarum specimen is now producing some form of insulin!!! I'm in the process of running a lot more tests to see if the insulin is fully developed or only partially, but I'll keep you posted! Essentially, we know it's expressing the DNA I put into it, so now we just need to find out if it's making the proteins exactly the right way, or if I need to do a little more fine tuning.
These last few months have had several major breakthroughs and we're now this much closer to creating a specimen that produces perfect insulin, then assembling the first PhyR machine prototype! Obviously so much more work has to be done and many more resources are needed, but we're doing it! Again, I cannot thank you guys enough for the donations, shares, support, love, and faith! This is possible only because of you and everything you do. I can't do this alone. So thank you thank you thank you!!! More updates to come! As always, if any of you have questions or want to hear the details, I'm always so down to talk about any of this.
Update - 06/17/20
We're still alive! Since last fall, the PhyR Project has won various awards in competitions at Brigham Young University, and Physarum and I were featured in an article the Utah Business Magazine!
Read the article here: https://www.utahbusiness.com/affordable-insulin-crisis/
We've kept up progress in our protein expression as well, and are several iterations along in the prototyping of the PhyR machine. Thank you all so much--this is only possible because of your continued support! More soon, and as always, feel free to reach out!